SARS-CoV-2, Vaccines, pandemic research and knowledge sharing by world loving people
Infection Tracker
COVID Worldometer - Covid19 daily infection update
Vaccine Tracker
Covid19 Vaccine Tracker - tracks vaccine candidates and development
COVID-19 Timeline
Coronavirus: A timeline of how the deadly COVID-19 outbreak is evolving
Using modern technologies which could involve development of hardware and software in different ways from vaccine development, testing, using AI for protein prediction, using electricity on cells, etc.
SARS-CoV-2 Genome Sequence of Spike (S) Protein
The virus is a Single-Stranded RNA virus. From the dissected genome sequence of the RNA the only part for creating S Protein is used for creation of the DNA/RNA based vaccines. The length of S Protein sequence from the virus is about 1274 codons/nucleotides-triplets. Modification of the sequence could be made to optimize the vaccine.
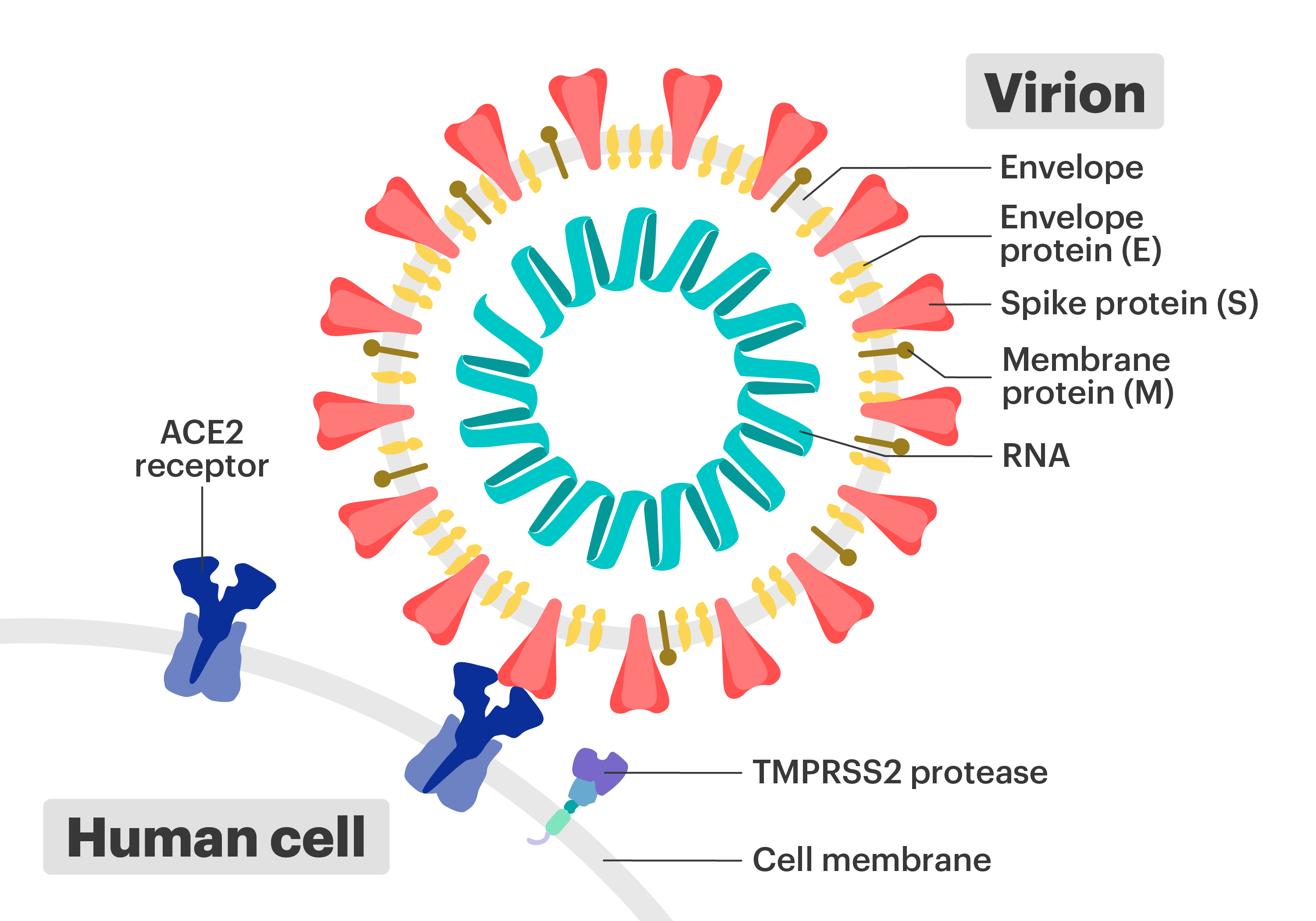
This virus generally binds with cells with ACE2 receptor on the cell.
RNA in Virus
(SARS-CoV-2, scientific)
DNA
Deoxyribonucleic Acid
Nucleotide/Nitrogenous base molecules contained:
A / Adenine
T / Thymine
G / Guanine
C / Cytosine
Chemical Structure of (common) DNA

RNA
Ribonucleic Acid
Chemical Structure
(single-stranded RNA vs double-stranded DNA)

RNA is a biopolymer, can be of a single/double/... strands. mRNA sequence can be derived from codons in a RNA or a DNA.
Nucleotide/Nitrogenous base molecules:
A / Adenine
U / Uracil
G / Guanine
C / Cytosine
A sequence of 3 Nucleotides makes a three letter codon. Codons gets read by Ribosome creating strand of Amino Acids. A strand of Amino Acids make a protein.
Adenovirus vaccine
The creation of vaccine which employs Adenovirus. This generally involves "reverse transcription" of SARS-CoV-2 S Protein RNA genome sequence and splice this into DNA polymer of the Adenovirus - creating the Viral Vector. From injection into the body, this Adenovirus enters the cell, taking this DNA into the cell's nucleus, this produces the mRNA, without ever touching cell's original DNA. This mRNA exits the nucleus and Ribosome reads the mRNA and starts to follow the codon sequence to create S Protein for triggering the immune response. The S Protein sequence could be tweaked to increase the effectiveness of the vaccine.

Approved by WHO:- Oxford/AstraZeneca: ChAdOx1 nCoV-19 / AZD1222 / Vaxzevria / Covishield, Janssen (Johnson & Johnson): Ad26.COV2.S/JNJ-7843673, Approved in some countries:- Gamaleya:(Ad26+Ad5) Sputnik V / Gam-COVID-Vac, CanSino: Ad5-nCoV, etc.
Note: Oxford/AstraZeneca target cells
mRNA vaccine
The mRNA made from genetic sequence of the S Protein is coated with Lipid Nanoparticle and this becomes the mRNA vaccine. The injection of this coated mRNA should enter the cell without triggering immune response. The mRNA never enters the nucleus. The Ribosome in the cell gets to work directly with the incoming mRNA, producing many S Proteins within the cell for triggering the immune response. Tweaking of the S Protein sequence in the vaccine could optimize the effectiveness. (This tweaking seems to be where the challenge is.)
Approved by WHO: Pfizer/BioNTech-- BNT-162b2, Moderna-- mRNA-1273
mRNA vaccine production (brief)
(of BNT-162b2, etc.)
The mRNA sequence of S Protein is inserted into Plasmid (small DNA molecule which can self-replicate) - making pDNA. This goes into a bioreacter for enzymatic reaction called In Vitro Transciption (IVT) by T7 polymerase and mRNA is produced. This gets purified and then coated with Lipid Nanoparticle.
Manufacturing Strategies for mRNA Vaccines and Therapeutics
mRNA Sequence
(For extensive info check: Bert Hubert section)
big Thank you
download
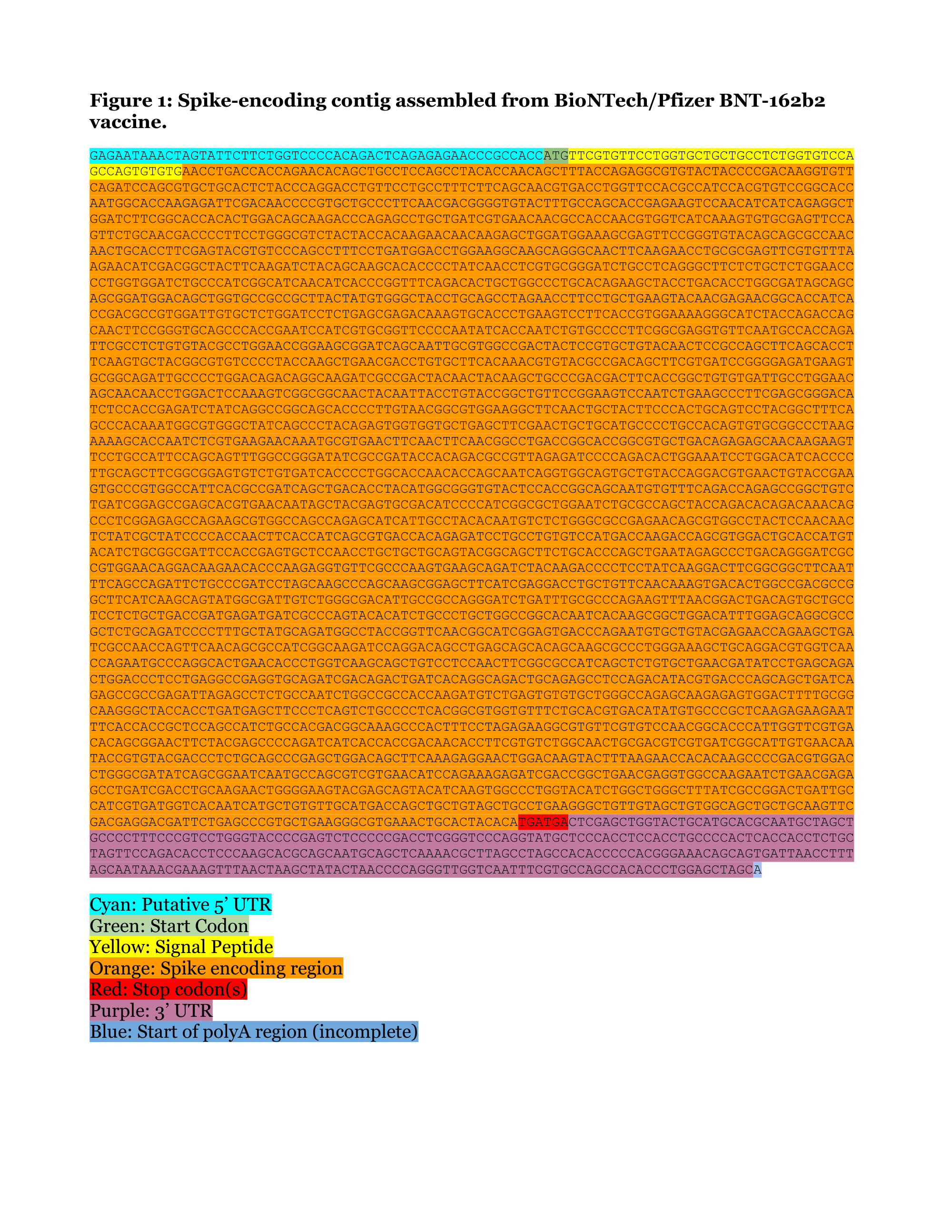
Assemblies of putative SARS-CoV2-spike-encoding mRNA sequences for vaccines BNT- 162b2 and mRNA-1273.
View
Pfizer/BioNTech BNT-162b2 - excerpt from Document
(substitute T with ψ)
Moderna mRNA-1273 - excerpt from the Document
(substitute T with ψ)

(Info from GitHub)
saRNA vaccine
Self-Amplifying RNA
This is a type of long-strand mRNA sequence containing the instructions to recreate itself (with creation of all necessary componenats for this production) as well as creating S Protein. With this technique, the amount of mRNA in the vaccine could be significantly reduced. (This technology still seems to be in trial phase.)
Lipid Nanoparticle
This is used for coating the mRNA and helps the mRNA gets into the cell. A Non-Specific Lipid Nanoparticle is used for delivering into general cells. A Lipid Nanoparticle with ligand is used for delivering mRNA to cells with certain type of receptor, this type is called Specific Lipid Nanoparticle.
Here are some methods in getting pDNA directly into the cell:
- Jet Injector - a needle-free injector which uses a narrow stream of fluid to penetrate the skin - Intradermal. (ZyCoV-D vaccine)
- Electroporation - electric pulse is used for getting the vaccine into the cell. (INO-4800 by Inovio - not yet approved)
ZyCoV-D vaccine
(by Zydus Cadila)
This vaccine has been approved under Emergency Use Authorization (EUA) by Drug Controller General of India (DGCI), it's the world's first approved DNA vaccine for COVID-19. It uses intradermal injection by using a jet injector to get the vaccine to penetrate the skin. The Plasmid DNA in the vaccine has the S Protein sequence. It is suitable for children from 12 years old or upwards. Three doses of the vaccine will be required (the trial for 2 doses will be conducted). Storage temperature is 2-8 degrees C with good stability at 25 degrees C for 3 months (according to their press release). The vaccine efficacy claimed is 66% from news (1,2).
Vaccination method
There are many ways to administer vaccine, different dosage volume, etc. There have been studies with the less common intranasal (IN - inhalation), intradermal (ID) injection, and also a relatively new pratice which uses intradermal needle-less jet injector. Some dose-sparing strageties have also been studied. Intranasal vaccines are also being studied.The intramuscular (IM) injection is the recommended method for BNT-126b2, mRNA-1273, AZD1222, Sinopharm, Sinovac, Covaxin, Sputnik-V and might also be for other vaccines. Intradermal needle-less jet injector is currently used by ZyCov-D vaccine from India's Zydus Cadila.
Few studies one dose-sparing (fractioned vaccine doses) in conjuncion intradermal injection strategy has been studied, addressing the issue of limited supply. (some studies might have not been peer-reviwed/published - excercise caution):
- (WHO) Interim statement on dose-sparing strategies for COVID-19 vaccines (fractionated vaccine doses) read (2021-08-10)
- Tolerability, safety and immunogenicity of intradermal delivery of a fractional dose mRNA-1273 SARS-CoV-2 vaccine in healthy adults as a dose sparing strategy read (2021-07-27 no peer-review)
- Intradermal Vaccination: A Potential Tool in the Battle Against the COVID-19 Pandemic? read (2021-03-06)
- Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces viral shedding after SARS-CoV-2 D614G challenge in preclinical models read (2021-08-11 orig: 2021-01-09)
Immune system and Vaccine

